Poster
4T1-luc2: An orthotopic mammary cancer model to support novel immuno-oncology drug discovery
Breast cancer is the second most deadly malignancy after lung cancer in woman in the United States, with an estimated 246,000 new cases and 40,450 deaths expected in 2016. Many treatment options for breast cancer exist including surgery, radiotherapy, anti-estrogen therapy, targeted therapies (e.g., trastuzumab), and chemotherapy. Despite these therapeutic advances, metastatic disease remains a significant source of mortality. Immunotherapy has seen remarkable progress in the last five years with four T cell immune checkpoint inhibitors approved. While none of these checkpoint inhibitors are approved in breast cancer, there are currently 60+ clinical trials ongoing in breast cancer with checkpoint inhibitors against PD-1 or PD-L1 and 20+ clinical trials ongoing with CTLA-4 inhibitors.
Because checkpoint inhibitors and other classes of immunotherapies require an intact immune system to elicit activity, syngeneic mouse models of mammary cancer are an important option for preclinical testing of novel agents. Labcorp has fully characterized the 4T1-luc2 mammary carcinoma model derived from Balb/c mice as an orthotopic model to support studies with novel immuno-oncology agents. Orthotopic 4T1-luc2 tumors metastasize to lymph nodes and the lungs making it a valuable model to study the effect of therapies on both primary tumor and metastatic disease using bioluminescence imaging. The growth of 4T1-luc2 tumors is equivalent in immune-deficient strains and immuno-competent Balb/c mice which suggests 4T1-luc2 is a weakly immunogenic tumor (Figure 1).
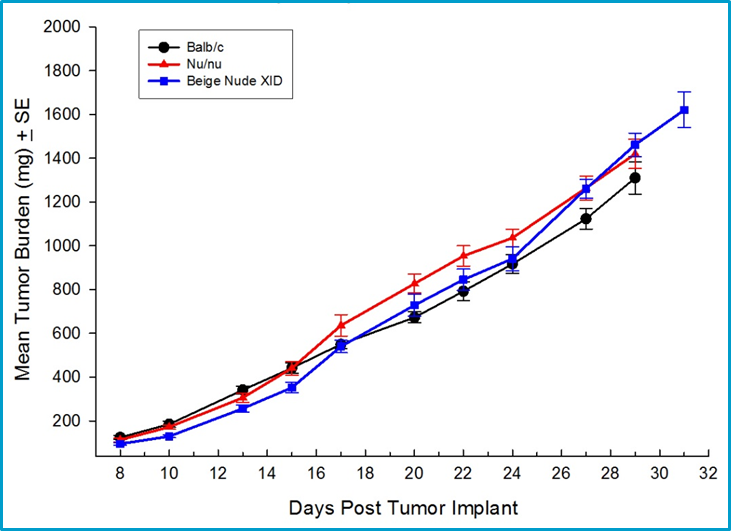
Fig. 1: Orthotopic growth of 4T1-luc2 in multiple strains of mice including syngeneic Balb/c mice.
Efficacy studies with antibodies to PD-1 or PD-L1 are hampered by a fatal hypersensitivity reaction in mice bearing 4T1 tumors.1 It is possible to give two doses without toxicity, but ongoing dosing is not possible. Instead, the efficacy of anti-CTLA-4 in combination with localized radiation was evaluated. A hamster anti-CTLA-4 antibody was used, and unexpectedly the hamster isotype control showed significant activity in some mice (Figure 2A), that was indistinguishable from the anti-CTLA-4 activity (Figure 2B). There was therapeutic benefit associated with localized radiation (Figure 2C), but no clear improvement with the combination of radiation and anti-CTLA-4 (Figure 2D).
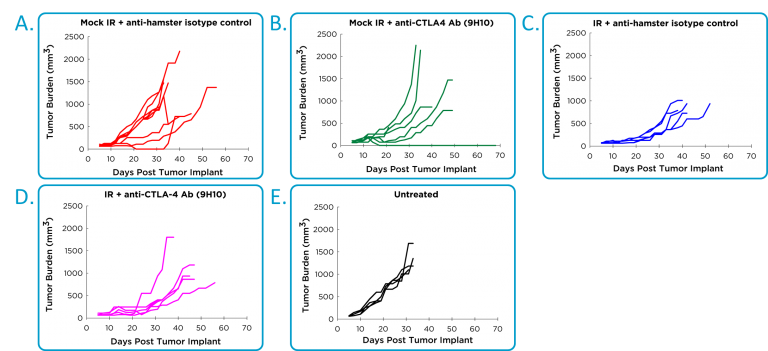
Fig. 2: Efficacy of anti-CTLA-4 and localized radiotherapy in orthotopic 4T1-luc2 mammary tumor model.
Metastatic burden was evaluated using bioluminescence imaging, and shown are representative images of metastases progression in untreated mice (Figure 3A). The anti-CTLA-4 treated mice had reduced metastatic tumor burden relative to the isotype control treatment mice (Figure 3B). Localized radiation also produced significant reduction in metastatic burden; however, there appears to be no improvement beyond the single agent activity of anti-CTLA-4 in the combination group.
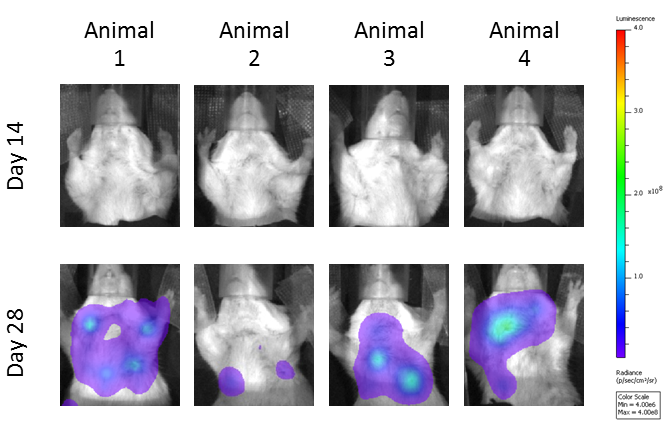
Fig. 3: Evaluation of total metastatic tumor burden over time by bioluminescence imaging:
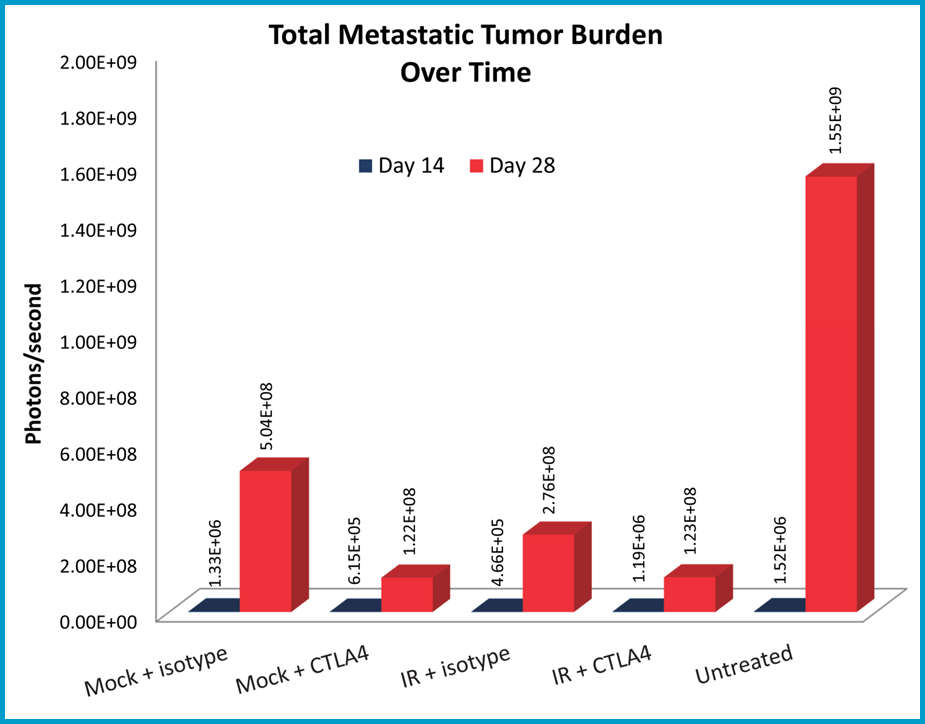
Fig 3B: Quantification of metastatic burden and response to treatment.
The radiation in this study was localized using lead shielding to reduce the total body radiation; however some animals still displayed signs of radiation toxicity. Labcorp now offers focal beam radiation capabilities using the small animal radiation research platform (SARRP) by Xstrahl which reduces systemic radiation toxicity.
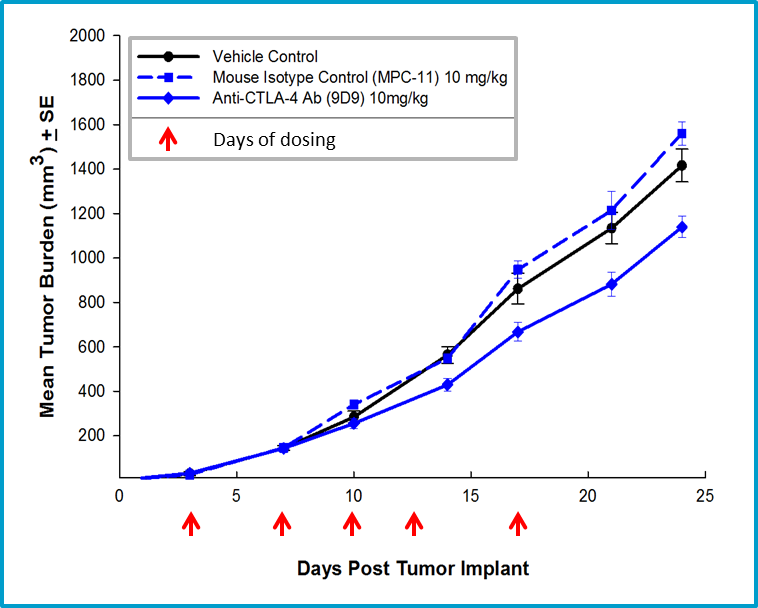
Fig. 4: Efficacy of anti-CTLA-4 therapy with mouse isotype anti-CTLA-4 antibody.
The non-specific anti-tumor activity associated with the use of a hamster antibody in the 4T1-luc2 model prompted a subsequent study using a mouse anti-CTLA-4 antibody. Mouse anti-CTLA-4 treatment resulted in minimal activity in the 4T1-luc2 model (Figure 4), but offers a potential combination partner for client sponsored studies with novel immuno-oncology agents. Further, we have extensive flow cytometry immune profiling of 4T1-luc2 tumors including quantification of CD4 T cells, CD8 T cells, regulatory T cells, granulocytic-MDSCs, monocytic-MDSCs, M1- and M2-tumor associated macrophages (TAMs) (Figure 5). The model is well characterized and available for client sponsored studies with novel immunotherapies or agents that may combine well with commercially available immunotherapies. Please contact us for further information and find out how Labcorp can help with your next study.
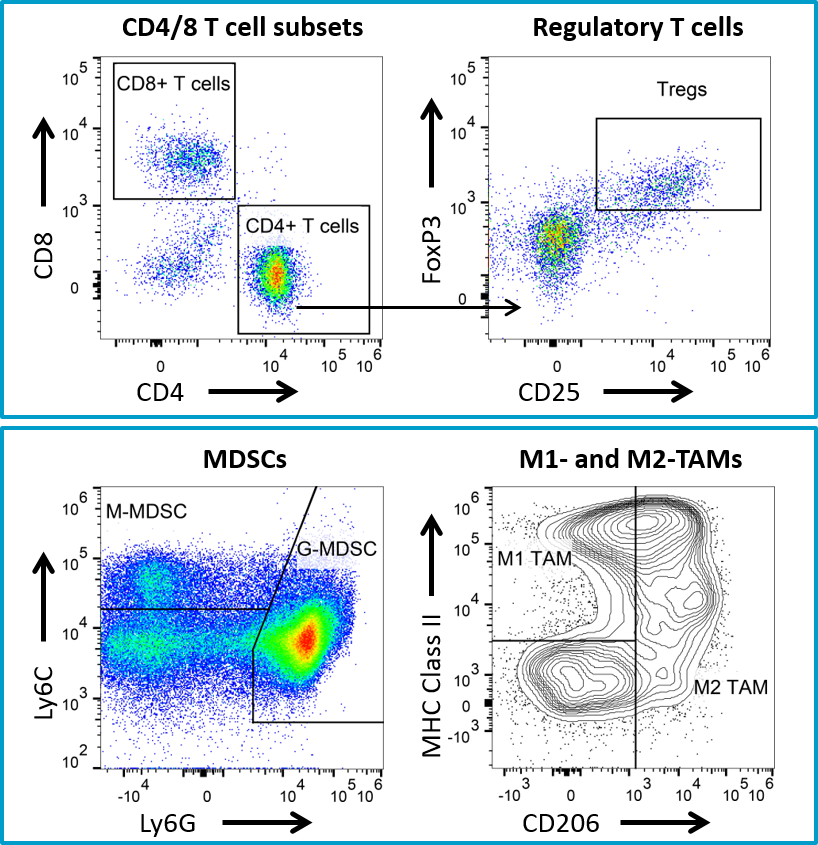
Fig. 5: Flow cytometry immune profiling for 4T1-luc2 tumors.
1Mall et al., OncoImmunology (2016), Vol. 5 (2): e1075114
Note: Studies were performed in accordance with applicable animal welfare regulations in an AAALAC-accredited facility


