01 Mar 2021
Author: Andrew P-A. Karalewitz, Ph.D.
Date: March 2021
Immunotherapy represents the state-of-the-art in cancer treatment. By harnessing the exquisite specificity of the immune system, modern immunotherapies can be designed to target a cancerous cell type while avoiding healthy tissue. Kymriah™ from Novartis and Yescarta™ produced by Kite/Gilead are FDA-approved cellular immunotherapies for the treatment of acute lymphoblastic leukemia and diffuse large B cell lymphoma respectively.
To produce Kymriah™ or Yescarta™, a patient’s T cells are engineered to express Chimeric Antigen Receptors (CARs) specific for the CD19 B lymphocyte molecule (1). With the recent clinical success of anti-CD19 CAR T cell therapies against blood cancers, the next generation of CARs with enhanced activity and safety are being designed.
Labcorp Preclinical Oncology (PCO) offers Chimeric Antigen Receptor (CAR) T Cell Generation. This service addresses the growing need for a reliable source of CAR T cells for use in early discovery studies. Sponsors may use pre-made anti-CD19 CAR T cells, or clients may provide lentiviral particles to express the CAR of their choosing using the Custom CAR T Cell Generation Service. Preclinical evaluation of multiple CAR candidates would allow sponsors to identify the most active CARs to progress through the drug development pipeline. The CAR T Cell Generation Service builds upon previous experience generating luciferase-expressing cell-lines and is complemented with in-house expertise in handling and culturing T cells and CAR T cells. Adequate quantities of CAR T cells for early discovery studies are produced with the CAR T Cell Generation Service.
The CAR T cell generation workflow is composed of three distinct technical phases: CAR T cell generation, flow cytometry to assess CAR expression, and an in vitro killing assay to test for CAR T cell activity. The in vitro killing assay may be used as a stand-alone service if sponsors already have a source of CAR T cells and are interested in testing CAR T cell function using one of the many cancer cell-lines available to clients. In this Model Spotlight, the CAR T Cell Generation Service is demonstrated using an anti-CD19 CAR as an example.
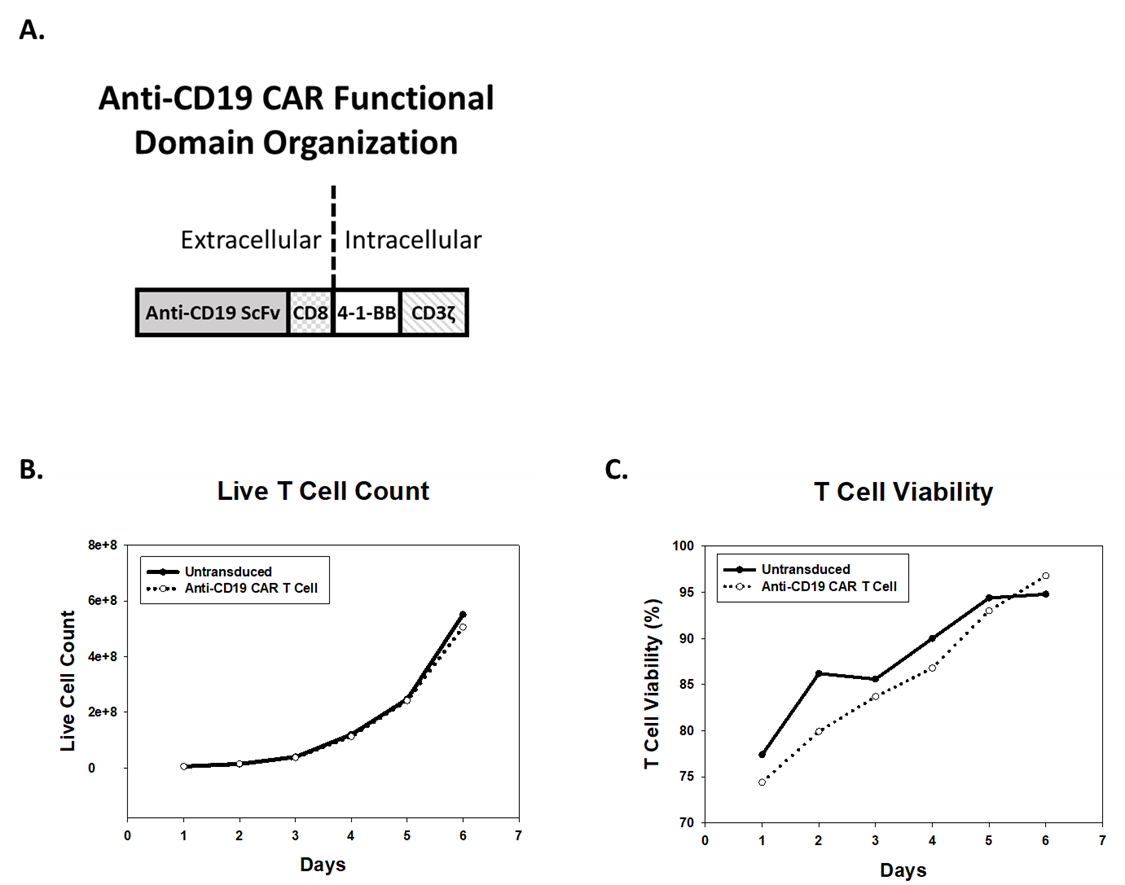
Anti-CD19 CAR T Cell Generation by Lentiviral Transduction
CARs are rationally designed proteins consisting of an extracellular antigen receptor fused to intracellular signaling domains. Antigen receptors are typically based on single chain variable fragments (ScFv) from a monoclonal antibody (mAb). Intracellular signaling domains consist of T cell-specific activity-modulators, such as 4-1-BB and TCR-ζ cytoplasmic signaling chain (CD3ζ) (Figure 1A) (1). To generate anti-CD19 CAR T cells, a lentiviral expression system is used to deliver genetic material encoding the CAR construct into T cells. Human peripheral blood mononuclear cells (hPBMCs) from healthy donors are transduced with lentivirus, expanded in culture, and subsequently cryopreserved for future use. T cells transduced with virus, or untransduced T cells (UTD), proliferate and achieve viability of greater than 90% post-transduction (Figure 1B and 1C).
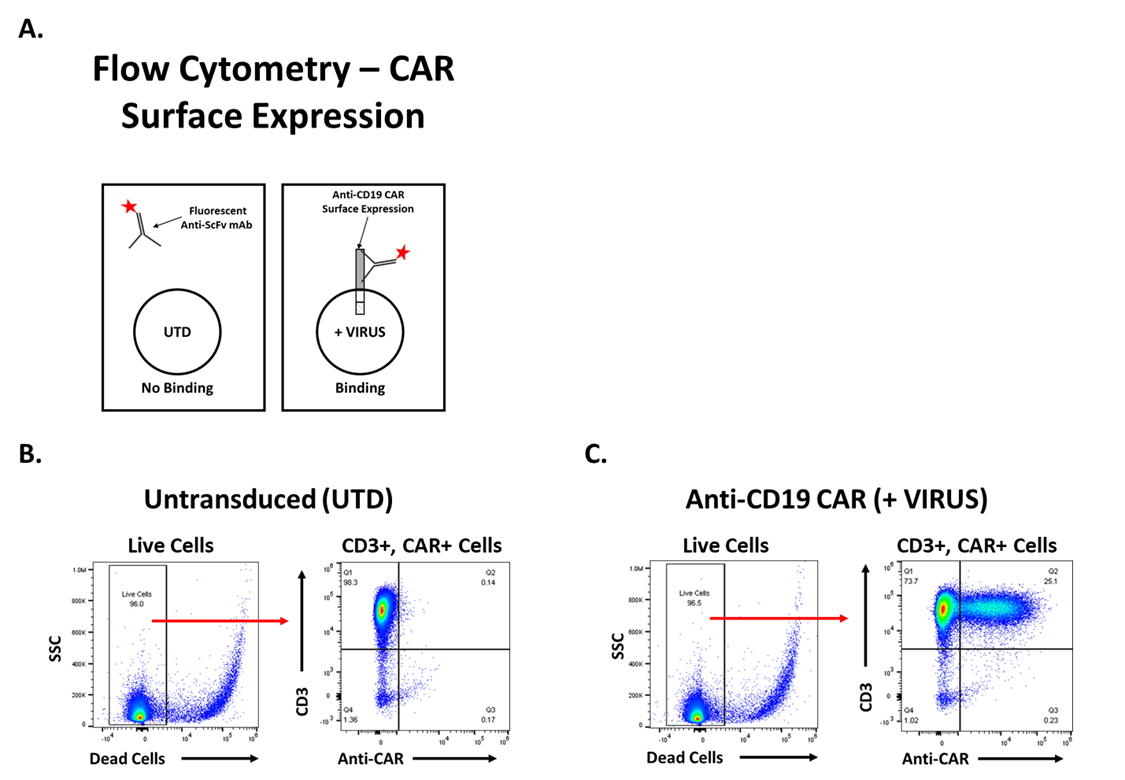
Assessment of Anti-CD19 CAR Surface Expression
Functional analysis of anti-CD19 CAR T cells is necessary to determine if lentiviral transduction was successful. Flow cytometry is used to quantify the number of CAR+ T cells and determine transduction efficiency. Surface expression of the anti-CD19 CAR is measured using a mAb that specifically binds the FMC63 ScFv (Figure 2A). Following gating to exclude doublets and debris, live cells are identified, and CAR expression is determined. Untransduced (UTD) T cells are 96% CD3+ and 0.14% CD3+/CAR+ (Figure 2B). After T cells are treated with lentivirus, approximately 25% of T cells are CD3+/CAR+ (Figure 2C). The increase in the percentage of double-positive T cells, CD3+/CAR+, indicates the transduction was successful. The UTD and anti-CD19 CAR T cells were both greater than 90% viable based on viability dye exclusion.
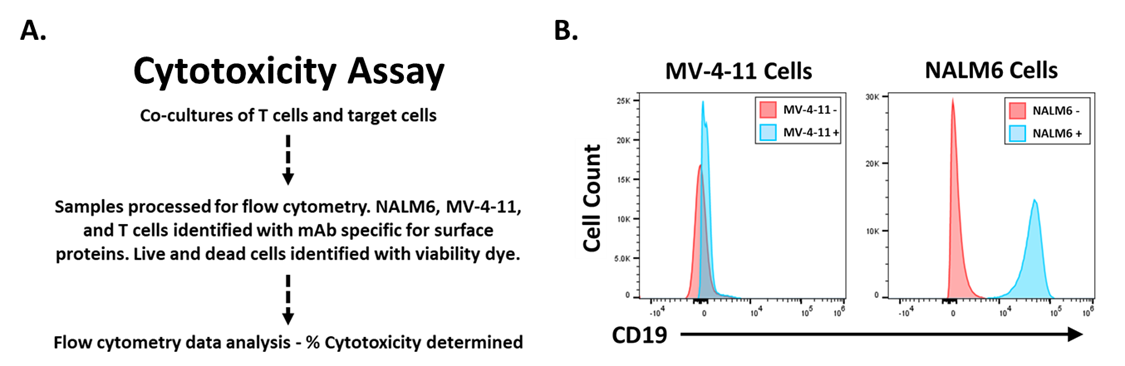
Assessment of Anti-CD19 CAR T Cell Function
To determine if anti-CD19 CAR T cells are functional, a target cell killing assay was conducted (Figure 3A). Briefly, target cells are co-cultured with UTD or anti-CD19 CAR T cells. The following day, target cell viability is measured by flow cytometry. Anti-CD19 CAR T cells should specifically kill target cells expressing CD19 while having no effect on target cells without CD19 expression. Flow cytometry to assess CD19 surface expression on target cells was conducted (Figure 3B). NALM6 cells express CD19, MV-4-11 cells do not (Figure 3B).
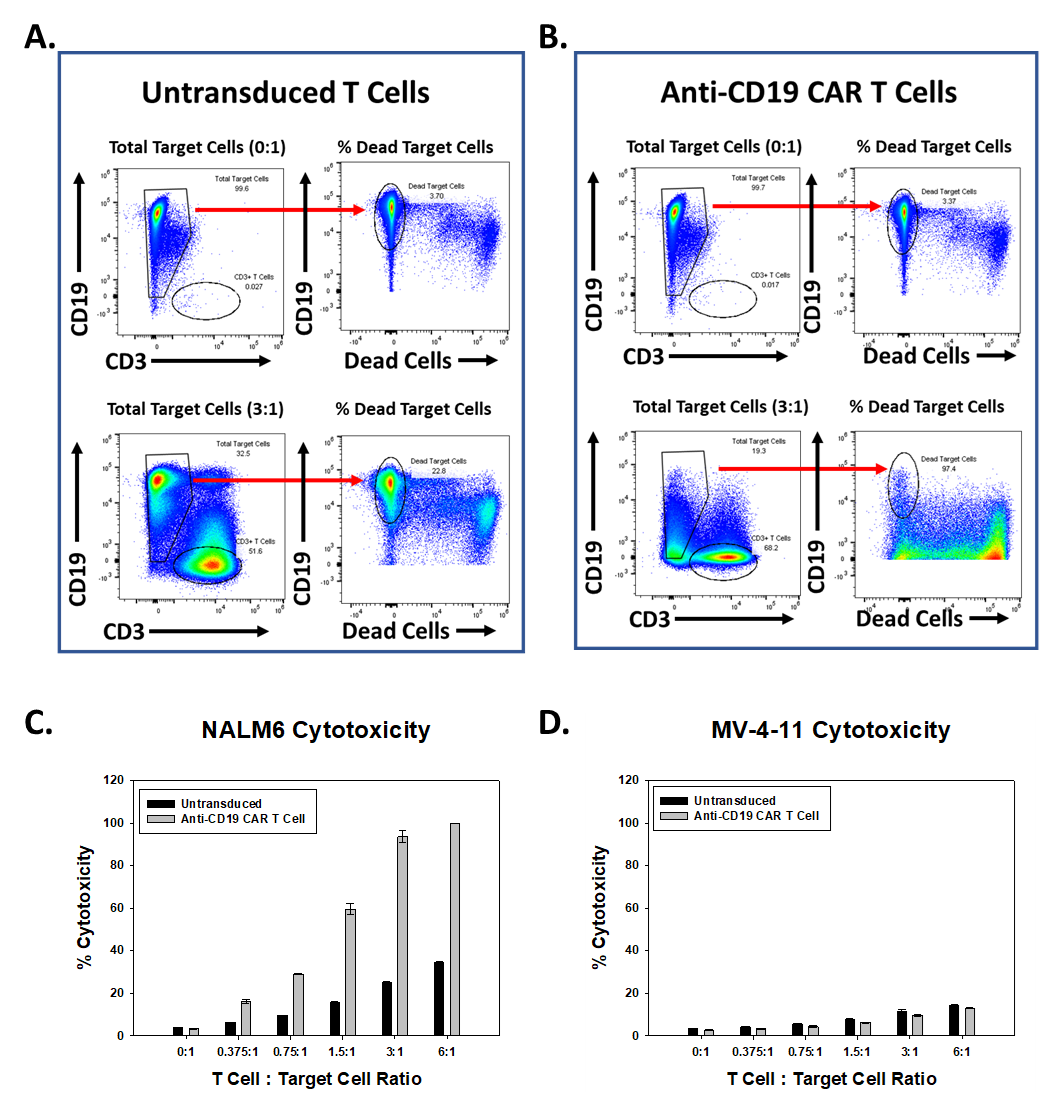
Anti-CD19 CAR T cells are cytotoxic towards NALM6 cells (Figure 4). Populations of CD19+ NALM6 cells and CD3+ T cells are identified. After gating on total CD19+ cells, the percentage of dead CD19+ cells was determined. Live cells are CD19+/viability dye negative. Target cells cultured alone were ~96% viable. Reverse gates indicate the percentage of dead target cells (3.7%) (Figure 1A). A greater percentage of dead NALM6 cells are observed in wells with anti-CD19 CAR T cells relative to NALM6 cells incubated with UTD T cells (Figure 4A, B, and C). The percent cytotoxicity towards MV-4-11 cells is equal for UTD and anti-CD19 CAR T cells (Figure 4D). The results indicate anti-CD19 CAR T cells are specifically active towards CD19-expressing target cells and verify the success of the CAR T Cell Generation Service
Summary
Starting with healthy, human PBMCs, Labcorp’s CAR T Generation Service produces CAR+ T cells in sufficient quantities for in vitro and in vivo characterization. The final deliverable produced with the CAR T Cell Generation Service described here are >95% CD3+, >90% viable and are specifically cytotoxic toward CD19-expressing human lymphoblastic leukemia cells. The T cell transduction protocol is based on established techniques and is amenable to alternative CAR constructs as well, allowing Labcorp to offer a Custom CAR T Generation Service to our clients (2). Generating CAR T cells for use in preclinical studies will advance our clients’ early discovery programs using combination or novel CAR T therapies.
