01 Dec 2020
Author: Patrick Allison, PhD | Scientist, Scientific Development
Date: December 2020
The advent of therapies harnessing the intrinsic anti-tumor activity of the immune system against cancer represents a paradigm shift in oncology therapeutics that has permanently changed the future of how we fight cancer.
Colorectal cancer (CRC) is a major contributor to the whole of cancer as a disease, representing 10% of all cases of cancer; over one million new cases of CRC were observed worldwide in 2018 resulting in over half a million deaths1. Clinical treatments involve surgical resection followed by adjuvant chemotherapy for non-metastasized colon cancer with more targeted therapies employed for metastatic CRC2. Recent clinical studies demonstrate strong results for immune checkpoint inhibition in the treatment of patients with unresectable, metastatic with high microsatellite instability (MSI-H), or mismatch repair deficient (dMMR) colorectal cancer3. Clinical benefit was so much so that Keytruda (monoclonal antibody targeting the human PD-1 immune checkpoint protein) was approved as a first line therapy for patients with CRC meeting these three criteria in the summer of 20204. With this new promise of immunotherapy against CRC, highly translational preclinical models of the disease are required to assess the effectiveness of novel immuno-oncology agents.
Assessing the efficacy of therapeutics targeting the immune system require preclinical tumor models with intact immune systems present for manipulation by test agents. Syngeneic tumor models represent such a strategy, in which mouse derived cancer cells are grown to form a tumor in the immune competent mouse strain of the tumor cell origin. We have extensively used CT26.WT murine colon carcinoma as a subcutaneous tumor model in female Balb/c mice to evaluate the efficacy of immuno-oncology agents - see our previous work in the model spotlights below (additional data available upon request):
- CT26 murine colon carcinoma spotlight
- Characterization of proliferation in multiple lymphocyte subsets in the CT26 murine colon carcinoma model by multi-color flow cytometry poster
- Immunophenotypic and Transcriptome Analyses of CT26 and 4T1-Luc Tumor Models Following Anti-mCTLA-4 Treatment poster
We recognize the experimental and translational limitations of subcutaneous tumor models of holding a different tumor microenvironment compared to tumors rising from the organ of origin including, but not limited to, disparate immune cell profiles that can affect response to immunotherapy5,6. Implant of tumor cells into the tissue of origin, known as an orthotopic tumor implant, seeks to rectify the limitations of subcutaneous studies and increase the potential translatability of the model.
In this model spotlight we will provide data that demonstrate a robust and reproducible orthotopic murine tumor model utilizing CT26.WT colon carcinoma, reliable surgical methods for implantation and validating response to checkpoint inhibition.
All animal work was approved by the site Institutional Animal Care and Use Committee and was performed in conformance with the Guide for the Care and Use of Laboratory Animals within an AAALAC-accredited program with humane euthanasia criteria predetermined on all studies.
Orthotopic Growth of CT26.WT-luc Tumors
Figure 1. Growth kinetics of CT26.WT-luc derived tumors orthotopically implanted in Balb/c mice
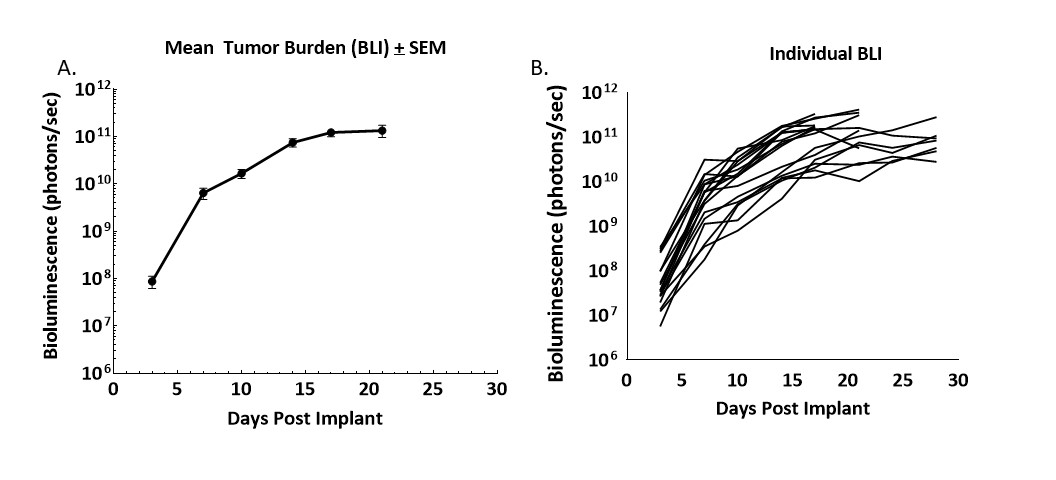
Figure 1 – Longitudinal monitoring of tumor burden following the orthotopic implant of CT26.WT-luc tumor fragments by bioluminescence imaging of 20 Balb/c mice. Bioluminescence was initiated three days following implant, group mean (A) and individual values (B) are presented.
Progression of tumor burden was robust and minimally variable. The tumor engraftment rate was 95% based on BLI and necropsy, no spontaneous regressions were observed. The BLI derived tumor volume doubling time (Td) was 1.5 days and the median time on study was 21 days.
Common clinical observations associated with disease progression were abdominal distension due to tumor growth and bodyweight gain (data not shown). Necropsy revealed large primary masses on the cecum and small nodules on the liver and abdominal wall. Primary tumors on the colon were observed to have large vascular integration with the colon indicative of efficient vascular recruitment by the tumor. Our next approach was to utilize this model for determining efficacy of immuno-oncology agents.
Checkpoint inhibition in the orthotopic CT26.WT-luc tumor model
Naïve Balb/c mice were subjected to surgical implant of CT26.WT-luc tumor fragments as described previously. Animals were staged by BLI three days following surgery and distributed into treatment groups based on BLI values. Animals were treated by intraperitoneal administration of 10mg/kg isotype control (clone LTF-2), anti-mPD-L1 (clone 10F.9G2), anti-mCTLA-4 (clone 9D9) or anti-mPD-1 (clone RMP1-14) twice weekly for two weeks. All antibodies were acquired from BioXCell (Lebanon, New Hampshire, USA). Animals were monitored by twice weekly BLI measurements for tumor burden, three times a week collection of bodyweights, daily clinical observations, and terminal necropsy observations.
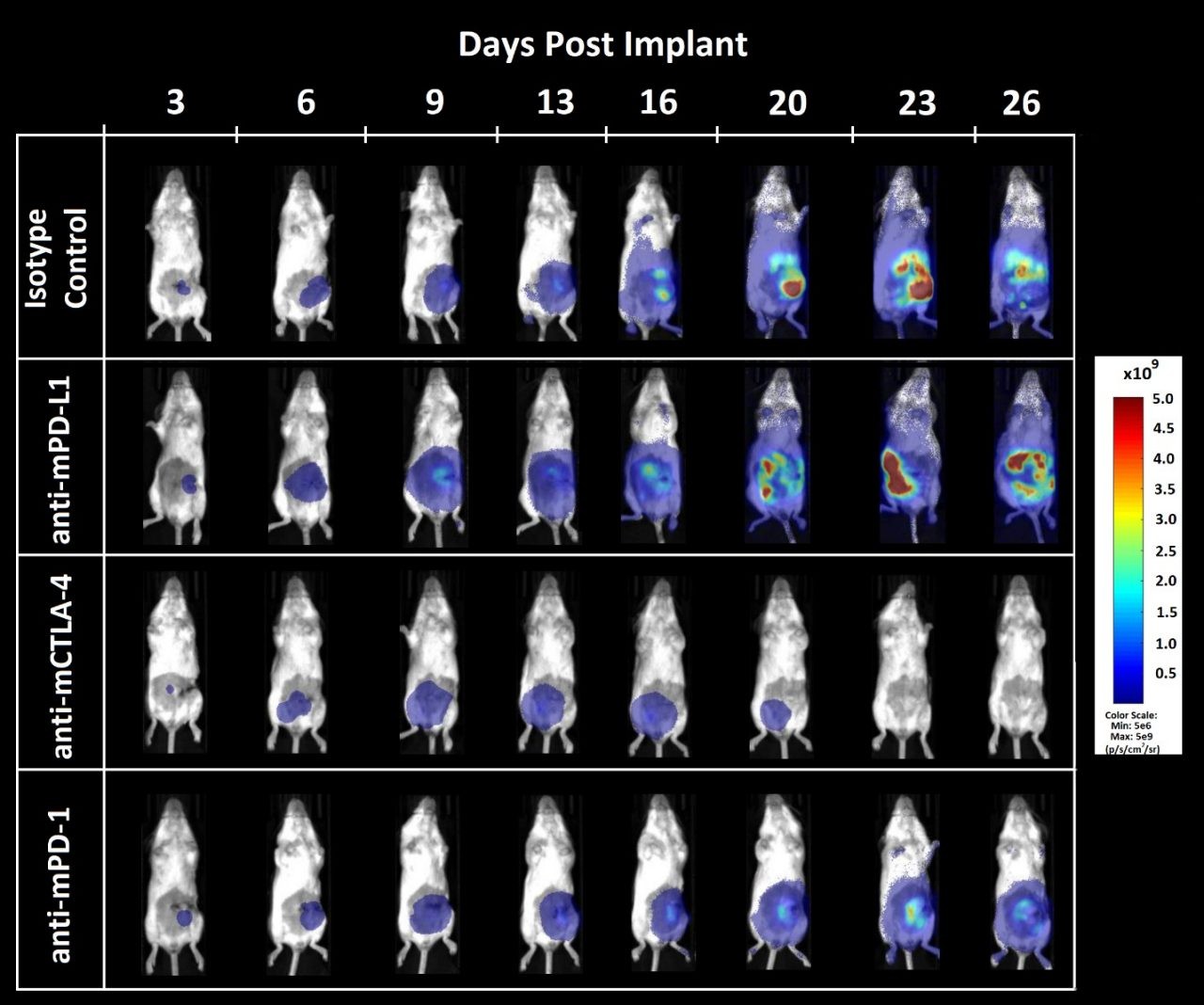
Figure 2 – Response of orthotopically implanted CT26.WT-luc tumors to checkpoint blocking antibodies. Group mean tumor burden assessed by BLI (A) and individual animal BLI values (B-E) in response to indicated therapies.
Administration of the isotype control antibody did not have an effect on tumor burden or disease progression (Figure 2A, 2B). Tumor doubling time of animals administered isotype control was 2.2 days, and the median time on study was 28 days. Administration of anti-mPD-L1 (Figure 2A, 2C) resulted in one complete regression (no detectable tumor burden above background BLI values). Anti-mCTLA-4 treatment (Figure 2A, 2D) resulted in a day 23 ΔT/ΔC of 0.1% and five partial regressions (BLI value less than half that of the first day of treatment) with no visibly detectable disease at necropsy. Administration of anti-mPD-1 (Figure 2A, 2E) did not result in regressions or tumor free survivors.
Figure 3. Bioluminescence imaging of CT26.WT-luc orthotopic tumor response to checkpoint inhibition
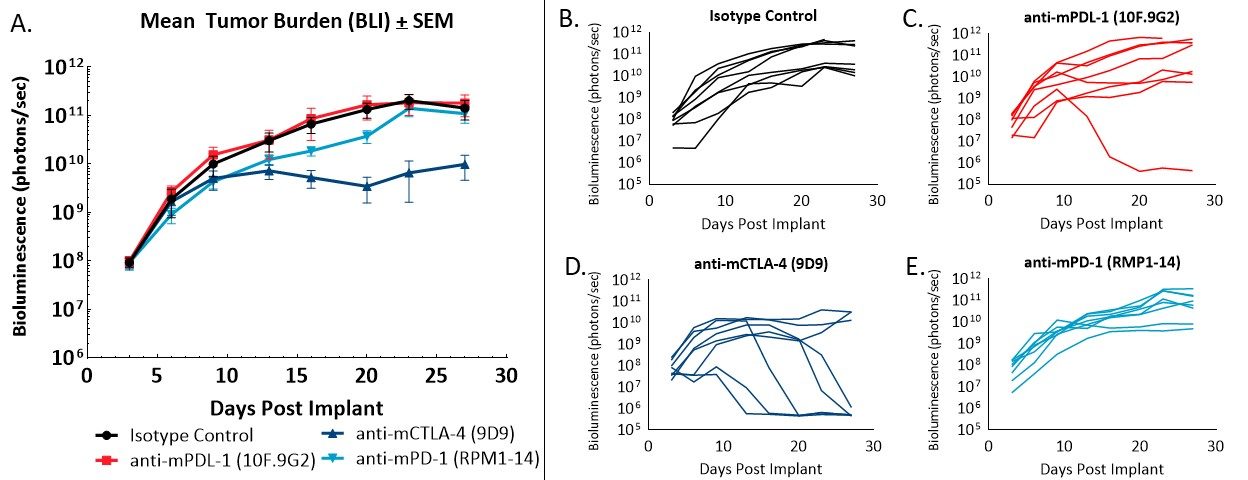
Figure 3 – Bioluminescence imaging of CT26.WT-luc derived tumors orthotopically implanted into female Balb/c mice treated with immune checkpoint blockade. Animals presented are representative of the median BLI value for each respective group.
Tumor burden was localized to the abdomen and increased over time (Figure 3) as expected based on previous pilot growth experiments (data not shown). Treatment with anti-mCTLA-4 resulted in delayed onset of disease related bodyweight gain (data not shown). Administration of anti-mPD-L1 or anti-mCTLA-4 provided an increase of life span benefit over isotype control of 12.5 and 23.6 days, respectively. Terminal necropsy revealed no evidence of tumors or other lesions in 1 out of 8 animals administered anti-mPD-L1, and 5 out of 8 animals administered anti-mCTLA-4.
Ongoing work is evaluating the differences between subcutaneous and orthoptic CT26.WT implant locations with respect to tumor immune cell infiltrate, histologic characteristics and morphology, along with immunologic activation in response to checkpoint blockade.
Taken together, we’ve demonstrated the CT26.WT-luc orthotopic model of colon cancer provides a robust platform for evaluating immuno-oncology agents in a highly translational implant location.
Contact our scientists to learn more about the CT26.WT-luc, and other murine orthotopic tumor models, and how we can apply these tools to advance your oncology pipeline.
