Articles
A20: Modeling B cell lymphoma in mice
01 Oct 2017
Researchers are using preclinical mouse modeling in an effort to employ conventional treatment methods for lymphoma, such as radiotherapy and chemotherapy, in combination with immunomodulatory agents to more effectively treat this disease. We have developed the syngeneic A20 tumor model, a B cell lymphoma, to support these immuno-oncology applications.
Lymphomas represent a highly heterogenous set of lymphoid cell malignancies. They can originate from either B or T cells, but B cell-derived lymphomas, specifically non-Hodgkin lymphoma (NHL), are the most prevalent. Lymphomas can be diagnosed in children and adolescents, but incidence of lymphoma increases significantly with age, the median age of diagnosis being 67 years. In 2017, an estimated 72,240 new cases of NHL in the United States will be diagnosed, and 20,140 patient deaths will occur. While the five-year survival rate for NHL is relatively high at 71%, recurrence can occur most often within five years of treatment,1 making the development of new treatment strategies for lymphoma essential for long term survival of these patients.
Given the impact of immunotherapy in other cancers, researchers are using preclinical mouse modeling in an effort to employ conventional treatment methods for lymphoma, such as radiotherapy and chemotherapy, in combination with immunomodulatory agents to more effectively treat this disease. We have developed the syngeneic A20 tumor model, a B cell lymphoma, to support these immuno-oncology applications. A20 was derived from a spontaneously arising reticulum cell sarcoma from an aging Balb/c mouse.2 A20 tumors are reported to express high levels of PD-L13 and are responsive to immunomodulatory antibodies. Taken together, A20 is an attractive model for use in immunotherapy drug development.
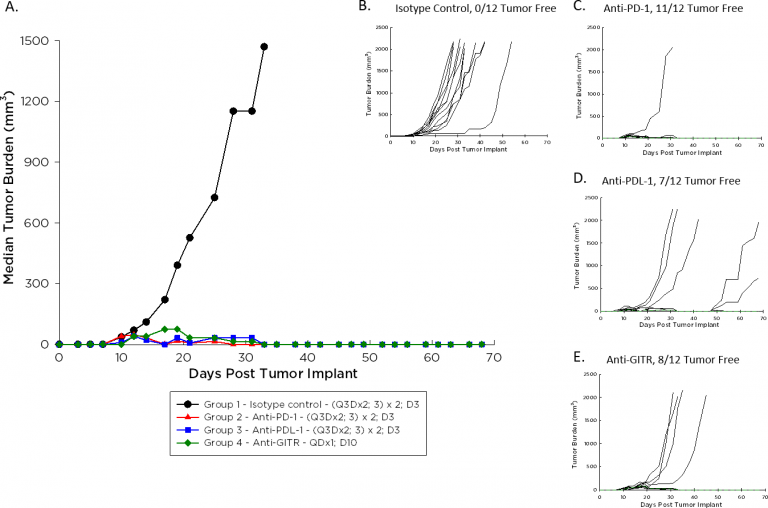
The doubling time of A20 is ~4-5 days, a moderate growth rate relative to other syngeneic models, potentially facilitating a longer dosing duration for test agents to elicit their anti-tumor activity. Figure 1 demonstrates median (1A) and individual (B-E) growth of control tumors compared to those treated with an immunomodulatory antibody. With dosing initiated on Day 3 after implant for the checkpoint inhibitors anti-PD-1 and anti-PD-L1, and on Day 10 after implant for the agonist anti-GITR, the anti-tumor response is striking. We expect that initiating dosing with these agents once A20 tumors are palpable will allow room for improvement in combination with candidate molecules.
Fig. 1: Median and Individual Growth of A20 Tumors Following Immunotherapy
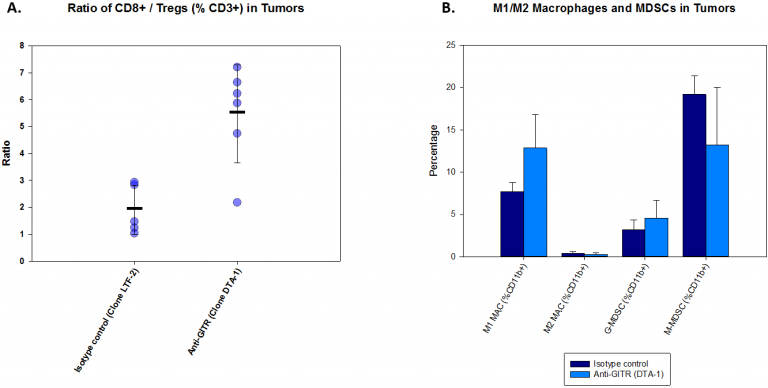
To investigate immunomodulatory effects of anti-GITR on the A20 tumor, we analyzed T cell and myeloid cell populations in tumor one week post-treatment. The results are illustrated in Figure 2. With anti-GITR treatment, the CD8+/Treg ratio increases dramatically (Fig. 2A). This increase indicates engagement of the host immune system which is also reflected in tumor burden decreases (Fig. 1). In contrast, changes in myeloid cell populations were modest. While some myeloid cell populations were unchanged with treatment, trends toward a decrease in %M-MDSC accompanied by an increase in %M1 macrophages are evident with anti-GITR treatment compared with isotype control (Fig. 2B).
Fig. 2: Immunoprofiling of A20 Tumors Following Anti-GITR Treatment
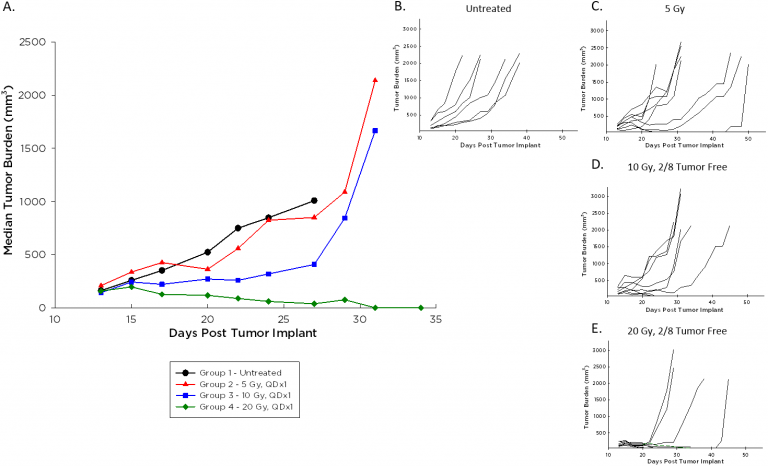
In addition to the promise of immunotherapy, radiation therapy is often used in the course of lymphoma treatment clinically and is typically administered in combination with other agents. We employ the Small Animal Radiation Research Platform (SARRP) by XStrahl for modeling focal beam radiation (see our related blog and poster), and investigated a single dose range of 5-20 Gy against A20 tumors. Median (Figure 3A) and individual (Figure 3B-E) tumor growth following this treatment are illustrated below. A20 tumors are impacted by radiation at all doses, and two mice each at the 10 and 20 Gy dose levels had durable complete responses almost 70 days post implant. These animals remained tumor free despite a rechallenge with a second injection of A20 cells, while tumors grew as expected in naïve age-matched animals (data not shown).
Fig. 3: Median and Individual Growth Curves of A20 Tumors Following Focal Beam Radiation
The A20 model provides robust preclinical means by which to study B cell lymphoma. The responsiveness of A20 to checkpoint inhibitors, costimulatory immunoagonists, and radiotherapy makes it an attractive model for use in the preclinical immuno-oncology space.
References
1Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
2Kim KJ, Kanellopoulos Langevin C, Merwin RM, Sachs DH, Asofsky R (1979) Establishment and characterization of BALB/c lymphoma lines with B cell properties. Journal ofImmunology, 122(2): 549?554.
